
Kanishk International Healthcare Group – an Independent Global Clinical Supply Chain (GCSC) Organization is an expert in pharmaceuticals, medical diagnostics/research/equipment, and ancillaries; with global capabilities differentiated by scientific leadership, innovation, and extensive networks. Our associations are operating in many regions.
K-Intl GCSC provides the full range of clinical trial services for pharmaceuticals, nutraceuticals, biopharmaceuticals, medical devices, diagnostic kits, and ancillaries for all trial phases, registration, and post-marketing trials. K-Intl GCSC is aimed around one objective i.e. accomplishing success for each Research Project.
Please request our Corporate Brochure accompanied by a Pharma Solid / Suspension Dosage Product List for Anti-HIV, Anti-TB & Anti-Leprosy therapy drugs, manufactured at the cGMP Facility, offered for export supplies. Kindly address your CT / Research inquiries to the undersigned at: admin@k-intl.co.in
K-Int’l GCSC offers a complete, integrated sample management solution that solves these issues, enabling improved study timelines with increased sample integrity and sample utilization which, ultimately, can help accelerate trial outcomes and patient health.
Our extensive experience in managing clinical trials spans all therapeutic areas, regardless of phase or size, within one global, interconnected platform. Our experts will work with you to customize our suite of services to fit your individual sample management needs.
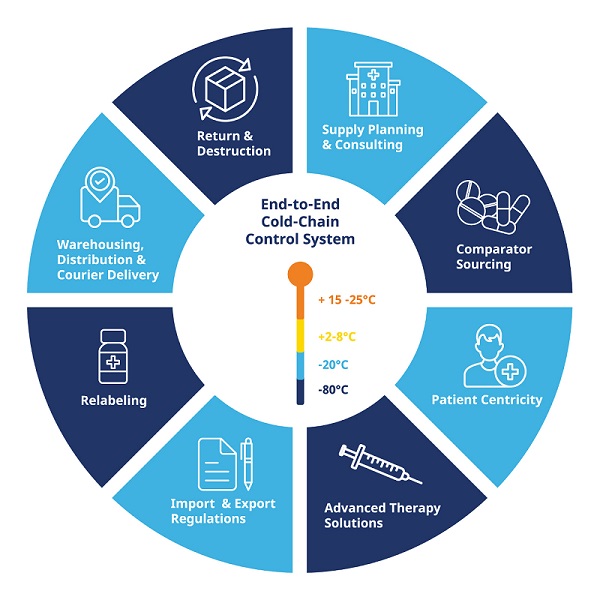
Whether you provide Investigational Drug Products to clinical sites or a Pharmaceutical company approved to commercialize a Final Drug Product, we can partner with you to deliver flexible, regulatory-compliant storage, and distribution solutions. Unrivaled expertise in ultra-low cold storage at temperatures ranging from -40oC to -80oC and LN2 and quick turnaround and real-time visibility of material inventory.
Time is of the essence. Partnering with highly qualified and experienced material management experts eliminates the need to train additional staff – or evaluate onsite overflow storage availability for temperature-sensitive material. We already have the infrastructure in place, and we are here to provide storage and distribution solutions to get your drug to market faster.
K-Int’l GCSC has logistics associates in many international locations to help you obtain product information, determine the right combination of technologies for your facility, or access K-Int’l GCSC Support.
K-Int’l is associated with Research Institutions facilitating ‘End-to-end’ supplies of Biologicals / Research Materials /Ancillary Supplies & Services to the pharmaceutical and biotechnology industries.
K-Int’l – is an innovation-led solutions provider that partners with the global pharmaceutical and healthcare industry to improve patient healthcare outcomes.
From pre-formulation to global logistics and distribution, our integrated offerings are aimed at establishing your critical success factor – OTIF. Our services bridge drug discovery with the manufacture of Clinical Trial Material with the speed and precision you need to accelerate market entry and drive profitability.
Our presence through our Strategic Business Partners in Americas, Israel, Europe, Japan, S Korea and Asia complements our extensive capability to service your ‘End-to Development and Manufacturing’ needs. Our Team and business associates include some of the best industry talents who combine knowledge and sensitivity to mitigate all regional complexities that could hamper both the quality and duration of trials.
At K-Int’l, our commitment to quality defines the path to project success. Our exclusive focus is to meet your strategic clinical / research trial needs with world-class capabilities and unwavering focus on service quality.
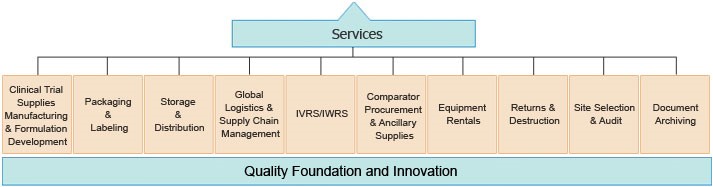
K-Int’l serves the global pharmaceutical and biotechnology industries with clinical / research trial materials support, services and complete project management. With best-in-class Bio-Pharma Cold Supply Chain & facilities in multi regions, K-Int’l and its SBUs delivers end-to-end solutions that support a drug through the entire clinical trial life cycle. Our services for solid, semi-solid, liquid, DEA (CI-V), DNA, Serums, Proteins, and biotech clinical trial materials (CTM) satisfy a broad range of requirements from pre-formulation research and development, manufacturing, analytical services and clinical supplies packaging and labeling to IVRS, QP services, controlled-temperature (cold chain) CTM storage, worldwide distribution and returns and destructions accountability.
Please solicit your enquiries by return email to: kgenix2000@gmail.com.